Product Details
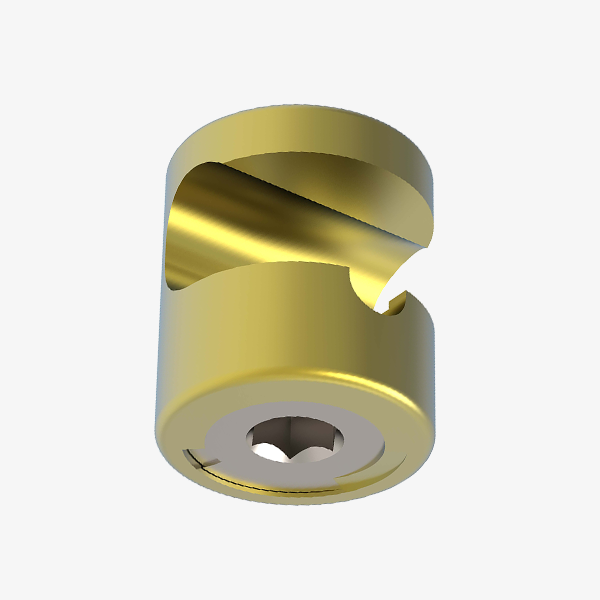
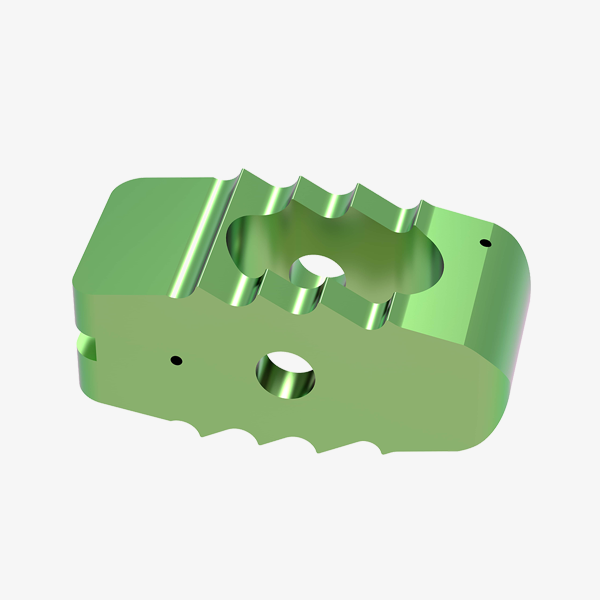
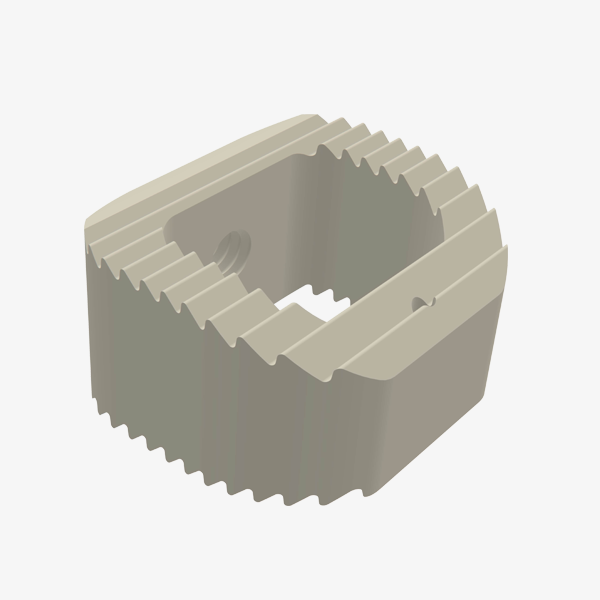
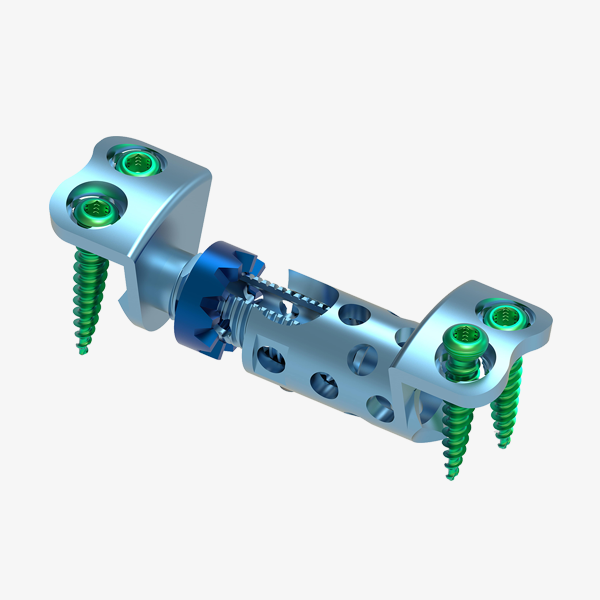
Polyaxial Reduction Screw ( 6.0MM ROD )
- Available in Dia 5.5mm & 6.5mm with length 30,35,40,45 & 50mm.
- Available in Titanium material.
- The system is indicated for use in the temporary stabilization or the anterior spine during the development of cervical spinal fusions in patients with degenerative disc disease, trauma (including fractures), tumors, deformity (defined as kyphosis, lordosis, or scoliosis), Pseudoarthrosis, and/or failed previous fusions.
- These devices are not approved for screw attachment to the posterior elements (pedicles) of the cervical, thoracic, or lumbar spine.
Other Similar Products.

Vegetables Juices
$149.00
$165.00
We want to give you 10% discount for your first order,
Use discount code at checkout

We want to give you 10% discount for your first order,
Use discount code at checkout